Not Boring by Packy McCormick - Medicine's Endgame
Welcome to the 1,563 newly Not Boring people who have joined us since our last Tuesday essay! If you haven’t subscribed, join 214,602 smart, curious folks by subscribing here: Today’s Not Boring is brought to you by… Percent Private Credit: It’s a popular asset class these days. You’ve maybe noticed an increase in headlines in WSJ, FT, etc. It’s a multi-trillion dollar asset class that’s grown 800% since 2006. But what is it? Pretty simple actually: Private credit is when non-banks lend money to private businesses who need capital. The only problem is that traditionally only institutional investors have access to investing in private credit. But now thanks to Percent, accredited investors can also access private credit with a minimum investment of only $500. Hi friends 👋, Happy Tuesday! Last week, we launched our new podcast, Age of Miracles. It went better than I expected: we’re currently top 20 in tech podcasts on Spotify and Apple. If you haven’t listened yet, check it out! The message seemed to resonate: we can live in an Age of Miracles, but it’s going to take a lot of work to get there. I’d like to be around (and healthy) to enjoy the fruits of all that labor. Cell-based therapy might be the most potent set of tools we have to make that happen. Cell-based therapies sound like science fiction. By engineering and reprogramming our own cells, we may be able to cure cancer, manufacture drugs inside our body, regenerate organs, and even reverse aging. While some of these applications remain in the realm of sci-fi, others are very real today. Doctors used a specific type of cell therapy — CAR-T — to cure a pediatric cancer patient for the first time in 2011. Cells are the fundamental building blocks of life. Humans are collections of trillions of them. Our Biotech Partner and Century of Biology author Elliot Hershberg believes that the ability to program our building blocks might be Medicine’s Endgame. There’s incredible progress being made in the field, and there are also immense challenges. In today’s piece, Elliot explores all of it with his characteristic blend of science, business, storytelling, realism, and techno-optimism. It’s ex-cell-ent. (sorry) Let’s get to it. Medicine’s EndgameWhat is a medicine? For most of us, medicines are small pills in white bottles with cotton tops. They have complicated names that evade quick recall and seem to get more expensive the sicker we get, but by and large they are miraculous. As a species, our collective medicine cabinet has swelled with increasingly potent and safe treatments over the past century. This has primarily been the byproduct of the industrialization of synthetic chemistry, which gave us small molecule drugs like statins that lower LDL cholesterol levels, reducing the risk of heart disease for millions of people around the world. Several decades ago, biotech fundamentally changed medicine by introducing a new wave of biological drugs—known as biologics—to treat diseases that small molecule pills couldn’t target. The first wave of biologics were primarily big molecules. Rather than stitching together short chemical structures synthetically, the discipline of molecular biology gave us a toolbox to produce much longer and more complex biologically occurring proteins at enormous scale. For example, if you’re a diabetic, a small molecule pill won’t do much good for you. The underlying driver of diabetes is insufficient production of a hormone called insulin that regulates blood sugar levels. The biologic for diabetes is simply insulin manufactured using an engineered cell. A little over a decade ago, the world of biologics was irreversibly transformed. For the first time, patients were cured of disease using genetically engineered cells as a medicine in their own right. If synthetic chemistry gave us an armamentarium of small molecules and molecular biology further expanded it with big molecules, the disciplines of cellular systems and synthetic biology are opening an entirely new frontier of programmable cell-based medicine. If we zoom out to the limit, cell-based therapies are likely to be the endgame of modern medicine. How can they not be? Every human starts out as a single cell. Through a profoundly complex cascade of genetically controlled cellular divisions, we ditch our microbial ancestors and grow into a mosaic of cells that are hierarchically organized into tissues and organs. Every disease stems from cellular dysfunction. By definition, if we can target—or even replace—diseased cells with other cells, it’s difficult to imagine a more effective medical paradigm. We’re talking about engineering a patient’s own immune cells to cure cancer, reprogramming cells and tissues to a younger and healthier state as we age, designing bacteria in our gut to pump out GLP-1 molecules when we eat too much, and curing diabetes with a single infusion of cells instead of countless infusions of insulin. If we continue to improve our capacity to program and produce cells, medicine will never look the same. The development of successful cell-based therapies was never a foregone conclusion. At several critical points, the field’s future hinged on individual patient outcomes where negative results could have sent the entire discipline into the academic backwaters for decades. Through a combination of perseverance, luck, and extraordinary scientific ideas, that didn’t happen. Cell-based therapy has achieved escape velocity and is now the focus of billions of dollars of investment around the world. Today, we’re going to explore the past, present, and future of cell-based therapies. We’ll look at what it took to get to where we are, how the first wave of cell-based therapies work, and how they are poised to redefine modern medicine from the ground up. The scope of cell-based therapies is almost biblical. If successful, these new medicines could cure cancer, mitigate blindness, and even reverse the process of aging. We may finally be en route to the future of medicine that sci-fi authors promised us. Engineering immunity to cancerFew humans operate with greater urgency than parents with sick children. This programming that Evolution baked into us has been a key driver of medical progress, especially for cell-based therapy. In 2010, this urgency was on display when Tom and Kari Whitehead faced a daunting set of decisions surrounding the care of their five-year old daughter Emily. Emily suffered from acute lymphoblastic leukemia (ALL), the most common form of childhood cancer. While decades of progress in cancer research had made the diagnosis less of a death sentence, Emily’s case was especially severe. After two rounds of chemotherapy, Emily’s illness seemed to only get worse. Her compromised immune system was unable to fend off a flesh-eating bacterial infection that was slowly destroying both of her legs. Amputation was a very real possibility that she narrowly avoided. She experienced a brief remission. About a year and a half after her last round of chemotherapy, Emily’s cancer returned. At this point, her doctors recommended a grueling bone marrow transplantation. Faced with the prospect of their daughter undergoing such a brutal treatment, Tom and Kari decided to look for a second opinion—they desperately wanted relief for Emily even if it meant a medical moonshot. As fate would have it, they ended up at Children’s Hospital of Pennsylvania (CHOP), where they learned that a scientist at Penn named Dr. Carl June was working on a new experimental cure for ALL. At the time, the proposed therapy—called CART-19—was truly the definition of experimental. CART-19 introduced an entirely new mechanism-of-action with no existing precedent, and the FDA hadn’t yet cleared it for a first-in-human trial. As a result, Emily was sent back to her local hospital where she received another round of intensive chemotherapy. Her cancer still worsened, and hospice was recommended. For Tom and Kari, losing their daughter wasn’t an option—especially when doctors at CHOP thought that a real cure might be within reach. Although they were dissuaded by their local doctors who felt a risky experimental therapy would only lead to more suffering, the Whiteheads enrolled Emily in a clinical study at CHOP. In this study, she became the first child to ever receive CART-19 therapy. It’s hard to imagine the level of stress the Whiteheads and their physicians endured. Medical progress proceeds in fits and starts, and its history is full of experiments with tragic outcomes. Only a decade earlier, an 18-year old named Jesse Gelsinger died in an experimental gene therapy trial at Penn, causing gene therapy trials around the country to be temporarily halted. Emily’s case couldn’t have gone more differently—eleven years later she is healthy and cancer-free.
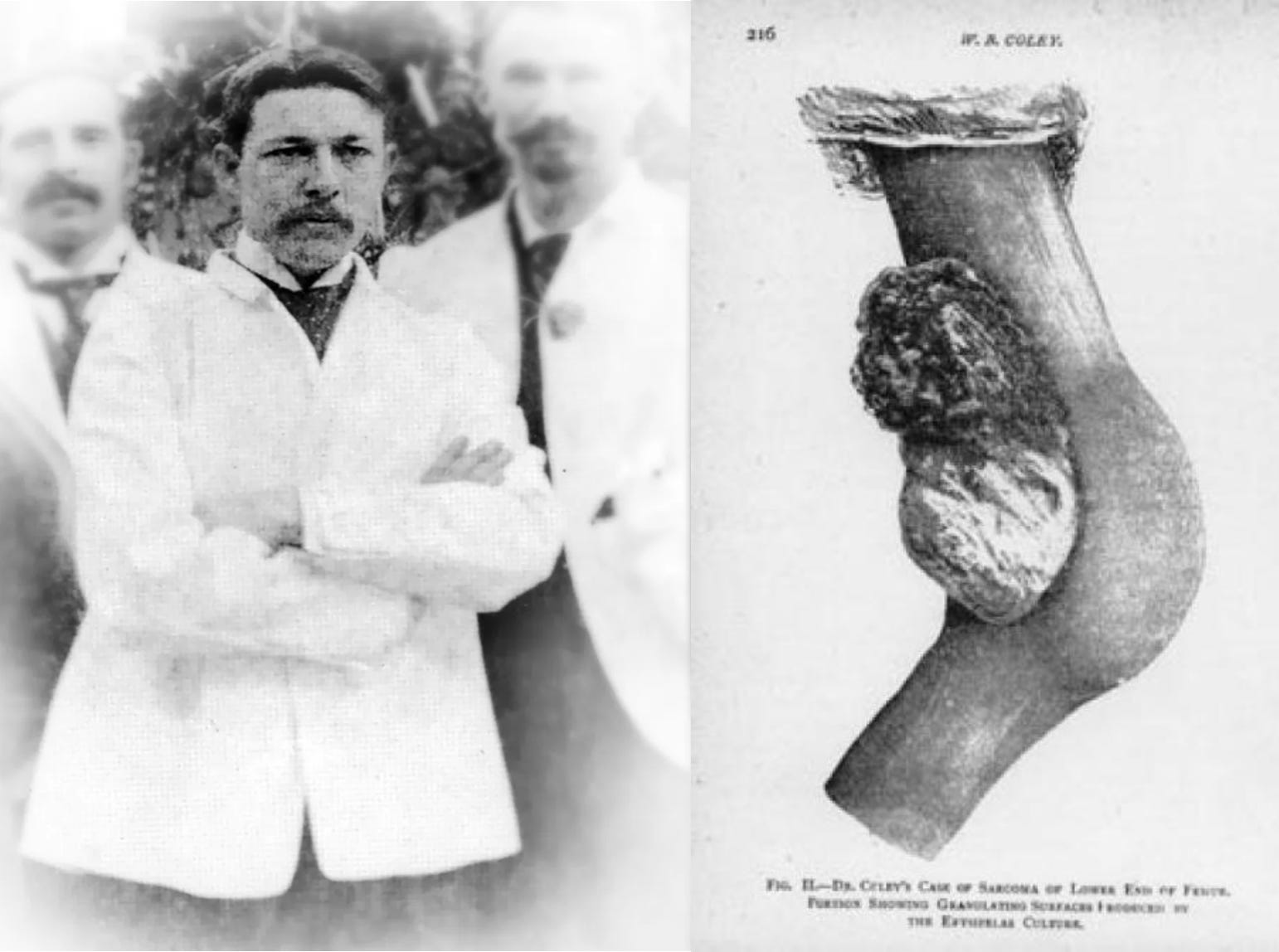
A therapy capable of pulling a child with terminal blood cancer back from the brink of death demands explanation. How does CART-19 work? CART-19 is a cell-based immunotherapy for cancer. The procedure involves extracting a patient's own immune cells and programming them using the tools of genetic engineering to target and destroy cancer cells once they are reintroduced into the body. While it reads like pure science fiction, the logic behind the therapy is grounded in over a century of compounding scientific observations and inventions. Towards the end of the 19th century, a surgeon named William Coley noticed a distinct relationship between infection and cancer that other physicians seemed to be missing. As he began his medical practice at New York Hospital, he struggled to treat patients with bone cancer who would die even after suffering through amputation to remove the original site of the tumor. One specific case rocked him. A young 17-year old girl named Bessie Dashiell presented with swelling in her hand—a closer examination revealed that it was bone cancer. Despite having her entire forearm amputated, Bessie died in a matter of days after her arrival at the hospital due to widespread cancer metastasis. Coley’s inability to offer any better treatment deeply affected him. He felt a sense of obligation to his patients to do better. After failing to treat Bessie, Coley combed through the case reports at the hospital for other cases of bone cancer. Through his review, he identified a case that seemed to defy explanation. Seven years before Bessie arrived at the hospital, a patient with an inoperable neck tumor was later discharged with no signs of cancer. The reversal of his disease couldn’t be attributed to any medical intervention—instead his cancer seemed to disappear after he caught a bacterial infection in the hospital. Obsessed with this observation, Coley experimented with a procedure that seemed to defy every tenet of modern medicine. For patients with inoperable bone cancers, he would inject bacteria directly into their tumors. As you can imagine, this was not an immediate success. Some of his first patients died of bacterial infection after injection. But for others, Coley observed otherwise inexplicable tumor shrinkage. The introduction of the microbes seemed to alert the body to the presence of the tumor. Over the course of his career, Coley continually refined the set of “toxins” he would inject into tumors, despite receiving harsh criticism from the medical community who argued his research program was an “entire failure.” History remembers Coley differently. He wasn’t wrong—the immune system is inherently capable of recognizing cancers and destroying them. Coley is now considered to be “the Father of Immunotherapy,” which is the modern medical field focused on stimulating the immune system to cure cancer.
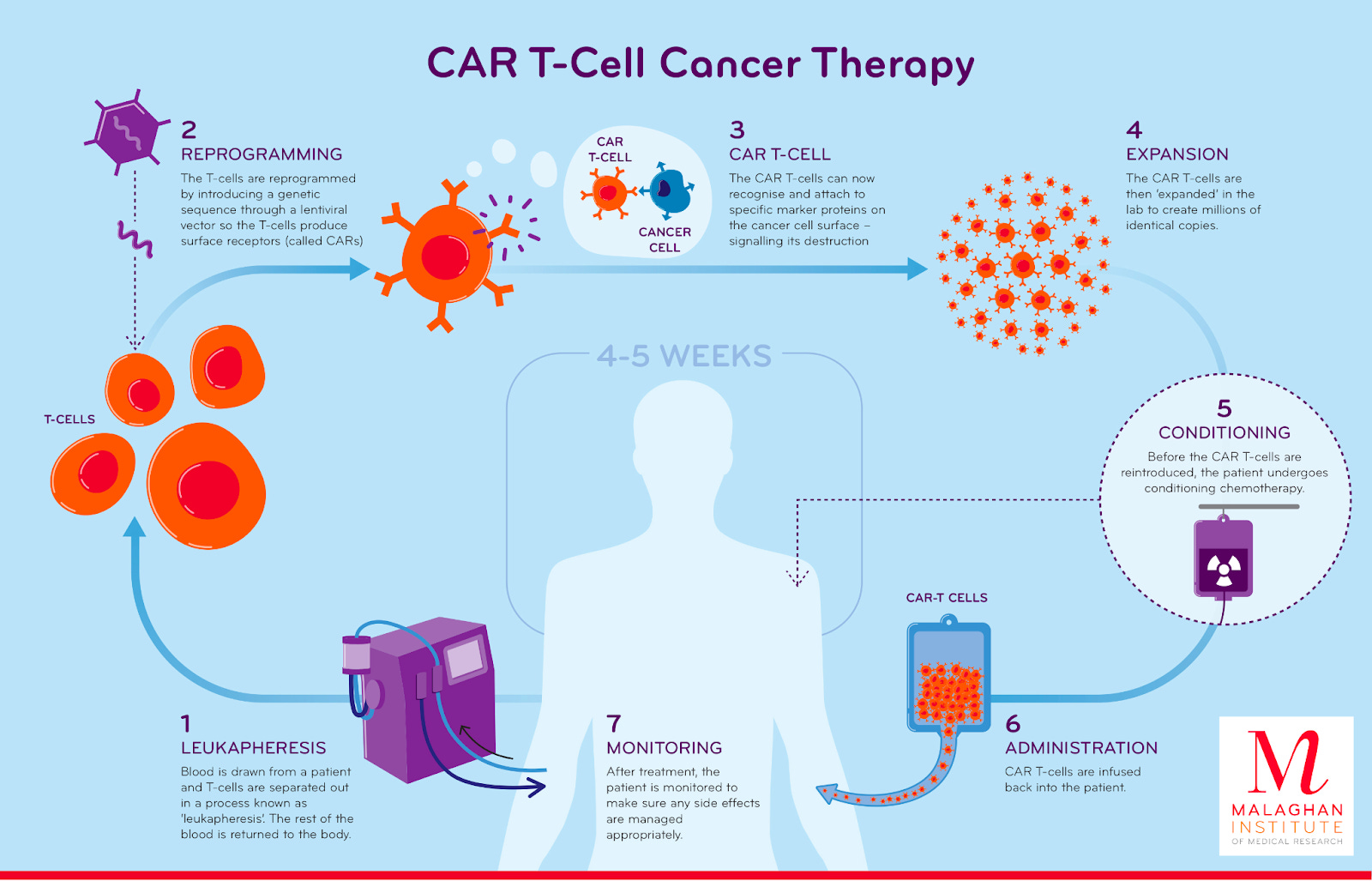
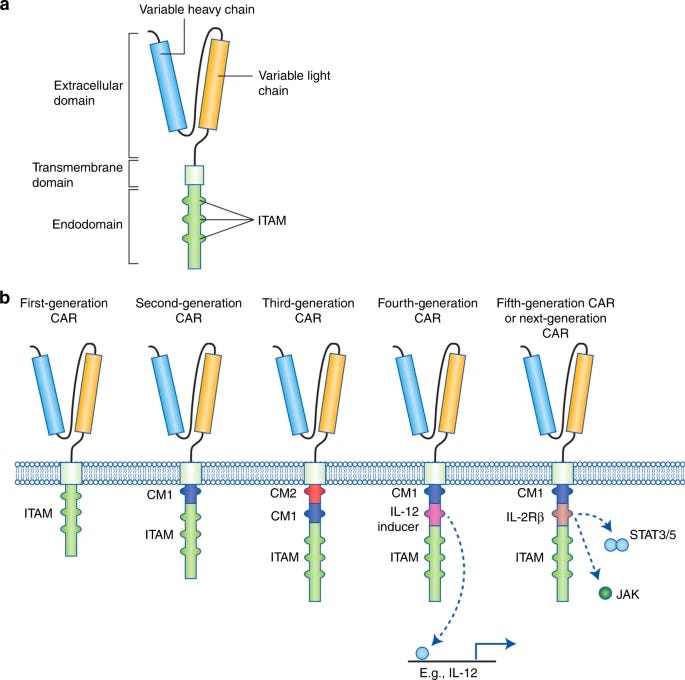
A century later, an Israeli immunologist named Zelig Eshhaar designed the type of cell-based immunotherapy that would ultimately save Emily’s life. The sophistication of the approach—called Chimeric Antigen Receptor T-cell (CAR-T) therapy—is a testament to how busy biologists were in the intervening years. Over the course of the 20th century, molecular biology and genetic engineering both emerged as serious scientific disciplines. Scientists resolved the molecular structure of DNA and developed a suite of tools for manipulating and transforming it. Far from Coley’s brilliant but crude “toxin” injections, Eshhaar was equipped with an entirely different language for how the body’s immune system battles disease. The emerging discipline of molecular immunology stratified the immune system into a complex typology of different cells, each with their own roles in the elimination of foreign bacteria and viruses. It became clear that a subset of white blood cells that migrated to the thymus—therefore called T-cells—were capable of exquisitely detecting foreign molecules called antigens. T-cells have receptors that bind specific antigens much like a lock that fits a specific key. Eshhaar’s fundamental insight was that T-cells could be genetically engineered to possess receptors that detected specific antigens. What if a T-cell was engineered to recognize a cancer antigen? This is what CAR-T therapy does. A patient's own T-cells are filtered out of their blood before being genetically engineered to express a T-cell receptor that is spliced together from different parts much like Frankenstein—hence the term “chimeric.” The new receptor consists of an antigen binder that is displayed on the surface of the cell and a set of intracellular signaling domains that dangle into the cell. The engineered CAR-T cells are then circulated back into the patient’s blood.
Although the concept for CAR-T is an elegant refinement of Coley’s approach to immune activation, it was totally unclear that it would work where it matters most: in patients. The immune system is a double-edged sword—it can cause life-threatening disease when it uncontrollably targets the body’s own cells. The new therapy needed a fierce clinical champion with a personal vendetta against cancer that resembled Coley’s. Dr. Carl June proved to be the man for the job.
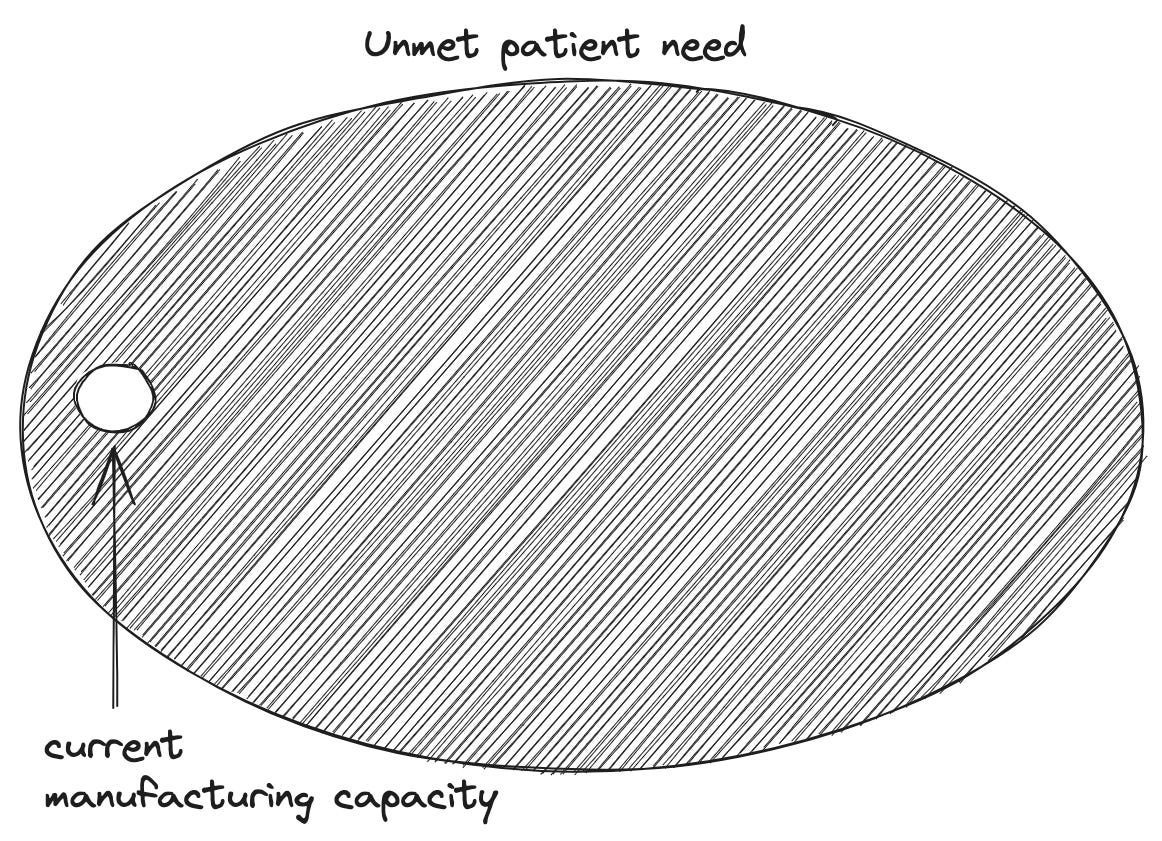
June’s motivation to improve cancer therapy came from a place of personal loss. He began his research career with a focus on procedures to boost immune function in patients with HIV. It was only when his 41-year old wife was battling ovarian cancer that he dropped everything to pursue cancer immunotherapy. The pivot was too late to impact his wife’s care, but her loss lit a fire that propelled him forward. There needed to be a better approach to treatment. Motivated by the preliminary designs and data around CAR-T, June launched the CART-19 study that Emily ultimately enrolled in. The name stems from the fact that the engineered receptors are designed to target CD19, a molecule only found on the surface of B-cells—which are the type of blood cells that are mutated in ALL. The result is a one-sided war between T-cells and B-cells where no B-cells are left standing. Over time, the body produces new B-cells that are cancer-free. The first patient doses—including Emily’s—got off to a rocky start. Just as had been predicted, hyper-stimulating the immune system with a foreign receptor was a risky business. After Emily’s third dose of CAR-T, she developed an intense fever, could barely breathe, and went into shock. For a moment in time, her life—and the future of this new procedure—hung in the balance. Emily’s lab orders were accelerated. With new data in hand, her doctors learned that her interleukin-6 (IL6) levels were off the charts. IL6 is a small signaling molecule that immune cells pump out to trigger cascades of inflammation. For a second time, illness in June’s family shaped the course of cancer immunotherapy. His daughter had recently been prescribed a medication targeting IL6 called Tocilizumab to manage her case of juvenile rheumatoid arthritis. June had a long-shot idea: what if they dosed Emily with Tocilizumab to curb the inflammation? In the eleventh hour, Emily’s prognosis reversed. Her shock quickly receded and her symptoms stabilized. Eight days later, a biopsy confirmed that the CAR-T procedure had been a success. To this day, her cancer is in complete remission. For Dr. Stephen Grupp, one of the essential physicians overseeing Emily’s care at CHOP, it seems to be a case of “the C word” that physicians can rarely use. Emily has seemingly been cured of cancer. Of the first three patients dosed with CART-19, two entered remission. Still, despite their extraordinary results, Dr. June and the Penn researchers reached another impasse. They were on the verge of running out of the philanthropic money used to treat the first patients, and knew they didn’t have the resources to statistically prove CAR-T’s efficacy. In 2011, June pushed the team to publish a pair of reports in The New England Journal of Medicine and Science Translational Medicine describing their initial findings. Finally, the world listened. The papers ripped across the biomedical community and the Penn team was approached by a wide range of investors and companies. Rather than starting their own company, June and his team chose the shortest possible path forward for CAR-T to enter the clinic. They decided to license the technology to Novartis, one of the world’s largest pharmaceutical companies, in exchange for $20M of upfront funding to manufacture CAR-T at scale for more studies. In 2017, Novartis made history by gaining approval to market the CART-19 medicine—now known as Kymriah (tisagenlecleucel)—for a subset of pediatric and young adult ALL patients. In doing so, Kymriah became the first FDA-approved gene therapy. The clinical data leading to its approval was nothing short of remarkable. In a multi-center trial with 63 patients, 83% were in remission within three months. Immunotherapy began with an observation about the relationship between foreign bacterial cells and disappearing cancers. Why not try to induce this response by systematically adding toxins to tumors? A century of compounding biomedical progress led to a very different outcome. Rather than introducing anything foreign into the body, the field achieved a medical first: a procedure to directly engineer a patient’s own cells to detect and treat disease. Kymriah was the first major “killer app” for the world of cell-based therapy. It represented a paradigm shift on the scale of what Genentech accomplished decades earlier. While Genentech used genetically engineered cells to produce medicines, CAR-T therapy genetically engineers cells for use as medicine. Paradigm shifts have a way of introducing entirely new sets of problems that require solving, much as scientific answers often lead to dozens of new questions to explore. It’s the perennial game of whack-a-mole that we call progress. For cell-based therapy, one of the most central new questions is: how can we deliver these new treatments for patients at scale? The looming manufacturing bottleneckWhen Emily was dosed with CAR-T therapy, it was still an academic procedure that required highly specific expertise. The entire workflow is far more complex than other medicines. Isolating T-cells from a patient's blood takes time, and the genetic engineering and downstream quality control is non-trivial. Despite the extraordinary initial clinical results, there were major open questions around turning CAR-T into a reproducible medicine. In some of the first studies, the cell engineering dragged on for months. In some cases, patients died before ever being dosed with the first batch of cells. By the time Kymriah was approved for broader use, Novartis had driven the turnaround time down to 22 days. While this represented a herculean leap forward in process engineering, the price tag for the procedure is still considerable: one dose of Kymriah costs $475,000. Part of this cost can be attributed to the fundamental need for drug developers to recoup their development costs—Novartis cites the fact that their total R&D spend exceeded $1B. A dose of Kymriah also represents something very different compared to other medicines: it is a potential cure for otherwise untreatable cancers. Still, a major question looms over the field. Can the time and cost of a cell-based therapy ever resemble other medicines? The current manufacturing process for this medicine requires transport of a patient’s cells—sometimes done via commercial flight—imposing a clear limit on achieving the speed and cost of a small molecule pill. First, a patient’s cells are extracted, frozen, and transferred to a central manufacturing facility, where they are engineered to express the CAR-T receptors and screened for quality. Next, they are frozen again and transferred back to the hospital where they need to be administered. This process barely satisfies the demand for the narrow clinical subset of patients that meet the criteria for the therapy. The manufacturing bottleneck is one of the major reasons why physicians are reluctant to recommend CAR-T therapy, and nearly a third of patients that are recommended for the therapy still never receive treatment. Clearly, this is a huge problem. Since the first approval for ALL, Kymriah has gained approval for two more types of blood cancer, and five other CAR-T therapies have entered the market. This is still only scratching the surface. Dozens of labs and biotech companies around the world are vying to create the first CAR-T therapies for solid tumors, which constitute nearly 90% of overall cancer diagnoses. If the status quo continues, CAR-T therapy could become a victim of its own success: it could remain a miracle cure that we can’t scale. Thankfully, The Techno-Capital Machine is already responding to this problem in two ways:
As Big Pharma companies have struggled to meet the demand for the first approved cell-based therapies for blood cancers, they’ve responded by ramping up their infrastructure spending. Just in the past year, BMS invested in a 244,000 sq ft expansion of their cell manufacturing facilities in Massachusetts, Novo Nordisk made a $136M investment in a cell therapy manufacturing hub in Denmark, and Bayer invested $250M in their own facility in Berkeley.
Sometimes, this type of large upfront capital expenditure (CapEx) can feel like a Field of Dreams moment: build it and they will come. David Cahn at Sequoia recently argued that NVIDIA’s GPU revenue has raised a $200B question for the field of AI: what products will emerge that are capable of generating a return on this scale of CapEx investment? This is not the case for cell-based therapy. There are patients dying before they receive the approved products that are already on the market. Pharma companies have to grapple with an even trickier problem: are the existing approaches to manufacturing scalable enough to solve the supply problem through investment alone? The answer is seemingly no. Again, if we’re en route to a future that encompasses solid tumor treatments, products for blood cancers are just the tip of the iceberg. And as we’ll soon see, cancer is only one disease category where cell-based therapy could soon emerge as the standard-of-care. It’s totally unclear that 200 more Launch Facilities will actually be able to meet future demand. Truly revolutionizing medicine with ubiquitous cellular medicines will require real invention. Going from Zero to One is the purview of startups, and a suite of new companies have emerged proposing futuristic solutions to the manufacturing bottleneck. Cellares, which recently closed a $255M Series C round and expanded their partnership with BMS, has built a new factory-in-a-box for cell-based therapy that they call the Cell Shuttle. Through a combination of hardware and software inventions, Cellares aspires to be an “Integrated Development and Manufacturing Organization (IDMO)” rather than a Contract Development and Manufacturing and Development Organization (CDMO) that meets demand by throwing existing technology and more humans at each new partnership. Whereas the new BMS facility required 500 new hires, technologies like the Cell Shuttle aim to produce the same volume of cell-based therapies with only 50. Carl June is hellbent on solving this problem—he’s an advisor and vocal proponent of Cellares, and has also co-founded his own stealthy CAR-T manufacturing company with $18M in backing from Danaher.
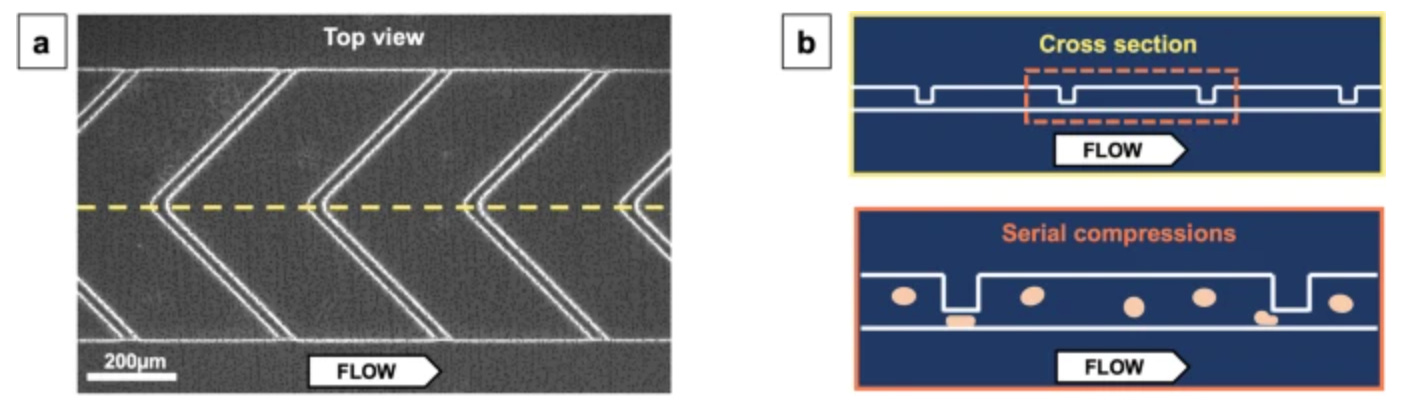
As if a network of robotic factories pumping out genetically engineered cellular medicines wasn’t a crazy enough idea, some startups are looking to bring manufacturing solutions even closer to the patient's bedside. A company called CellFE just raised a $22M Series A led by M Ventures (the venture arm of Merck KGaA) to continue to develop their microfluidic systems for cell manufacturing. The technology works by flowing cells along a tiny microfluidic chip where they are compressed as they flow through a narrow channel. When they re-expand, the molecular payload used to reprogram them flows into the newly available intracellular space. According to the founder, the vision is for cell-based therapy to evolve into a two-day process that is “manufactured and administered similar to what is done with dialysis today.”
Taken together, these investments paint a picture of several distinct manufacturing strategies vying for supremacy in a rapidly growing market. One possible future closely resembles the present: Big Pharma invests in a growing number of centralized factories that produce cell-based therapies much like we do now—round-trip flights included. Alternatively, Cellares and its competitors envision a future where we dramatically shrink the footprint of the existing factories using robotics and software, making it possible to distribute them much closer to hospitals around the world. The third and most extreme solution is to invent processes that circumvent the need for factories altogether. Imagine CAR-T therapy evolving into a routine outpatient procedure administered as soon as a cancer is detected. Ultimately, the final balance between these different candidate solutions will be determined through market competition. As Marc Andreessen argues, “The techno-capital machine makes natural selection work for us in the realm of ideas.” The selection function here is simple: which technology and business model can deliver the cheapest, fastest, and safest cell-based therapies to patients in need? CAR-T began its life as a controversial academic idea. Now, it’s an approved product with potentially curative effects for each blood cancer patient that is dosed. The major open questions are ones of scaling and engineering—the scientific question of efficacy has been answered in the clinic. This is a real breakthrough moment for the field of cell-based therapy. The thing is, CAR-T only scratches the surface of the full vision for cellular medicine. Let’s turn our attention to the biomedical frontier, where a whole host of engineered cell therapies are poised to make the same leap that CAR-T did: from concept to practice. The broader arsenal of cell-based therapiesIn 2013—only a year after the pair of papers describing the initial clinical CAR-T results—three faculty members from the University of California, San Francisco (UCSF) published an article laying out a vision for cell-based therapeutics as a “third pillar” of modern medicine alongside small molecules and the first wave of biologically derived big molecules. In their estimation, CAR-T was the first of four proposed “killer apps” for cellular medicine. The four applications were:
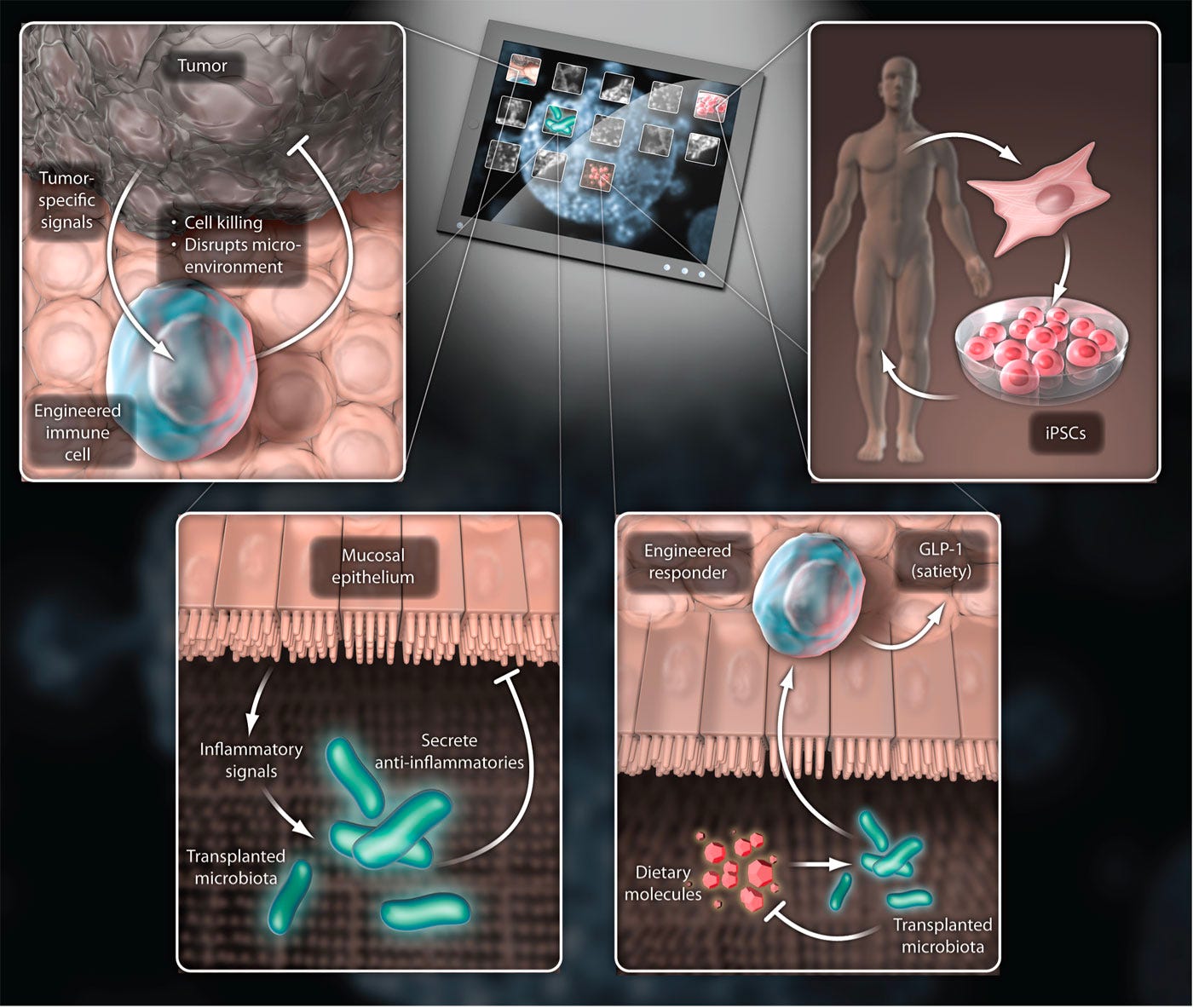
These predictions have fared well so far. As we’ve seen, CAR-T therapy has demonstrated that directly engineering immune cells is a viable therapeutic strategy. Still, CAR-T represents only one possible cell type, and one possible receptor. In a recent essay on immune engineering, I argued that this represents only a tiny sliver of the possible design space. For starters, there are a lot of other immune cells to engineer. Already, we’re seeing the engineered CAR receptor start to be ported to other immune cell types. Natural Killer (NK) cells—a well-named cell that specializes in targeted cell killing similar to T-cells—have also been equipped with CARs. The same is true for macrophages, another type of immune cell that is capable of gobbling up entire cells like a molecular Pac-Man. This generalization of the technology across the immune system highlights a way that cell-based medicine differs dramatically from the world of small and big molecules: reusability and composition is possible. One small molecule binds to one cellular target, period. A synthetic receptor resembles a piece of biological software—it is a genetically encoded module that carries out the same function in any cell that it is integrated into. What other biological software can we write? One clear direction is to design better CARs with more bells and whistles—another distinct benefit of working in genetic design space where iteration is much more tractable than in chemical space. The names of these new programs illustrate the evolution at work. For example, T-cell Redirected for Universal Cytokine-mediated Killing receptors—or TRUCKs—encode a molecule called IL-12 that serves to signal to other cells that they should activate into cancer killers. From CARs, to more heavy duty TRUCKs, engineered immune receptors will continue to be refined over time by genetic engineers.
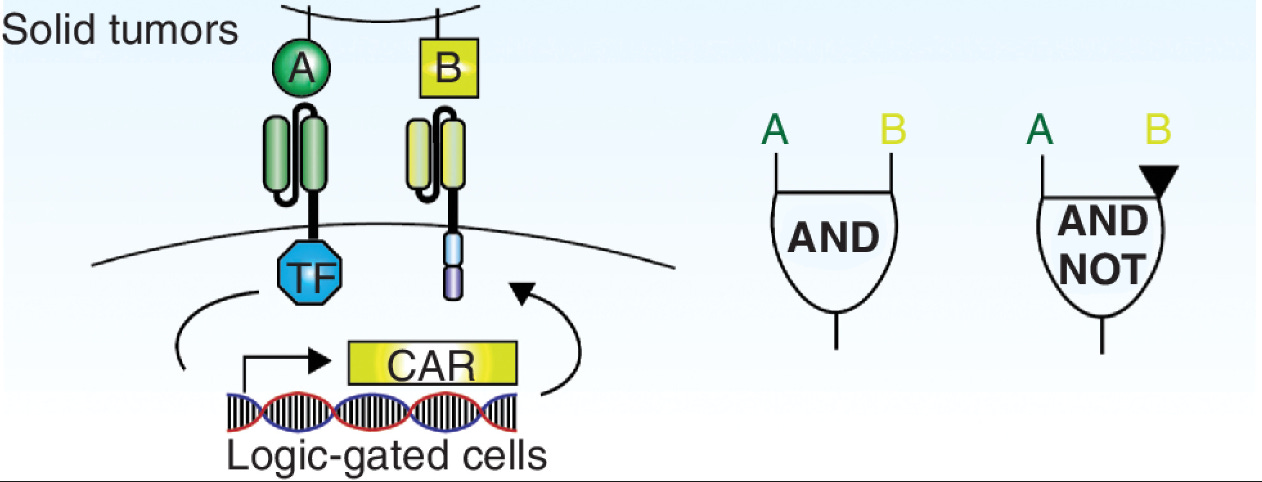
Another massive benefit that cells provide is the ability to program logic operations. Rather than sensing and binding to a single antigen, the design space extends to the world of genetic circuits that integrate multiple signals before triggering a response. SynNotch receptors rely on the activation of a cellular AND operation—A AND B need to both be present to run the CAR-T genetic program. This type of cellular computation will likely play a central role in the extension of immune engineering from blood cancers to solid tumors.
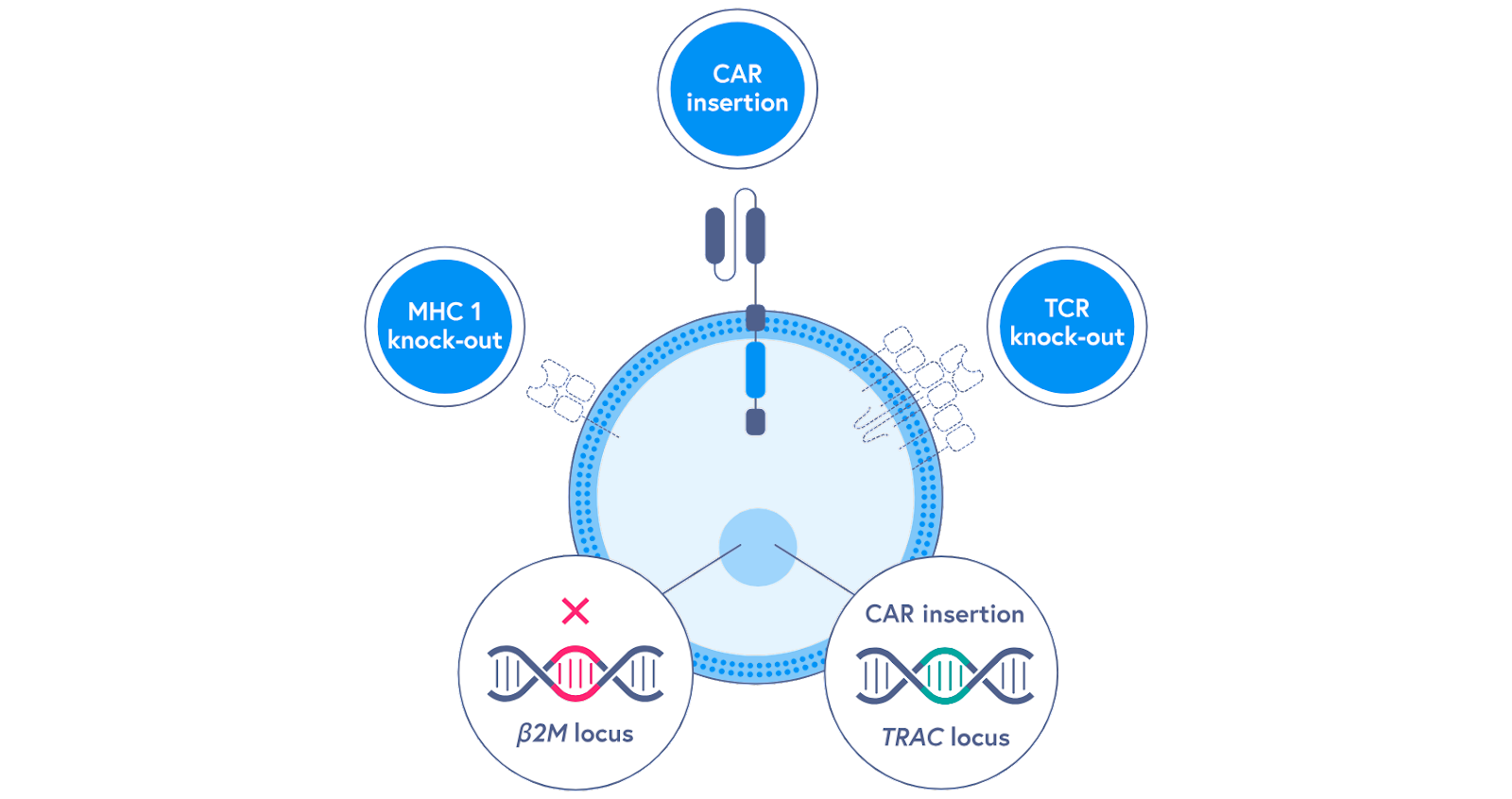
As the frontrunner for successful cellular medicine, immunotherapy also serves as a test bed for how we actually deliver the biological software that we write into a patient's cells. The current paradigm of extracting T-cells from the blood, engineering them, and then reintroducing them is called ex vivo cell therapy—which is Latin for outside the body. As we’ve seen, this approach introduces a serious scaling limit on clinical adoption of cell-based therapies with our current manufacturing infrastructure. What if we could engineer immune cells that we could store in biobanks like other biologics, and then use them when we need them? This type of “off-the-shelf” approach is referred to as allogeneic cell therapy, because it requires infusing a patient with cells that aren’t their own. Much like organ transplants, the major roadblock for allogeneic cells is the threat of graft-vs-host disease, where the body’s immune system recognizes the cells that are introduced to the blood as foreign and responds with a frontal assault—which can be life threatening. Several labs and companies are actively exploring ways to engineer donor cells to evade the immune system. Achieving this is a hard genetic engineering problem. It would require knocking out two sets of immune receptors, while simultaneously inserting the CAR sequence. A key breakthrough that makes this type of multi-edit design potentially feasible is CRISPR—a gene-editing technology that received the 2020 Nobel Prize for making it possible to programmably edit the genome. One thing that makes all of this especially mindblowing is that the seminal CRISPR paper was published in 2012, right on the heels of the first CAR-T papers. In one calendar year, we saw field-changing advances for cell-based therapy (the CAR-T papers in 2011), gene-editing (the publication of CRISPR gene-editing in 2012), and AI (the AlexNet moment for deep learning in 2012). 8VC partner Francisco Gimenez refers to it as the “Annus mirabilis for medicine” much like how much Einstein transformed physics with his four publications on the photoelectric effect, Brownian motion, relativity, and mass-energy equivalence all in 1905. It’s a beautiful example of Intertwining Threads, as Packy would put it: “One of the things that makes the future so hard to predict is that new technologies don’t operate in silos; they’re intertwining threads that weave together in unpredictable ways.” We’re seeing this property on full display in the life sciences: some of the first CRISPR companies such as Caribou Biosciences and CRISPR Therapeutics are pushing the hardest to make allogeneic cell therapies a reality. Next-generation T-cell engineering companies like Arsenal Bio are building extensive machine learning infrastructure to accelerate the construction of new synthetic cell modules. It’s a story of compounding exponentials—these inventions and discoveries don’t exist in isolation, they bleed into each other and are remixed in new ways to accelerate progress.
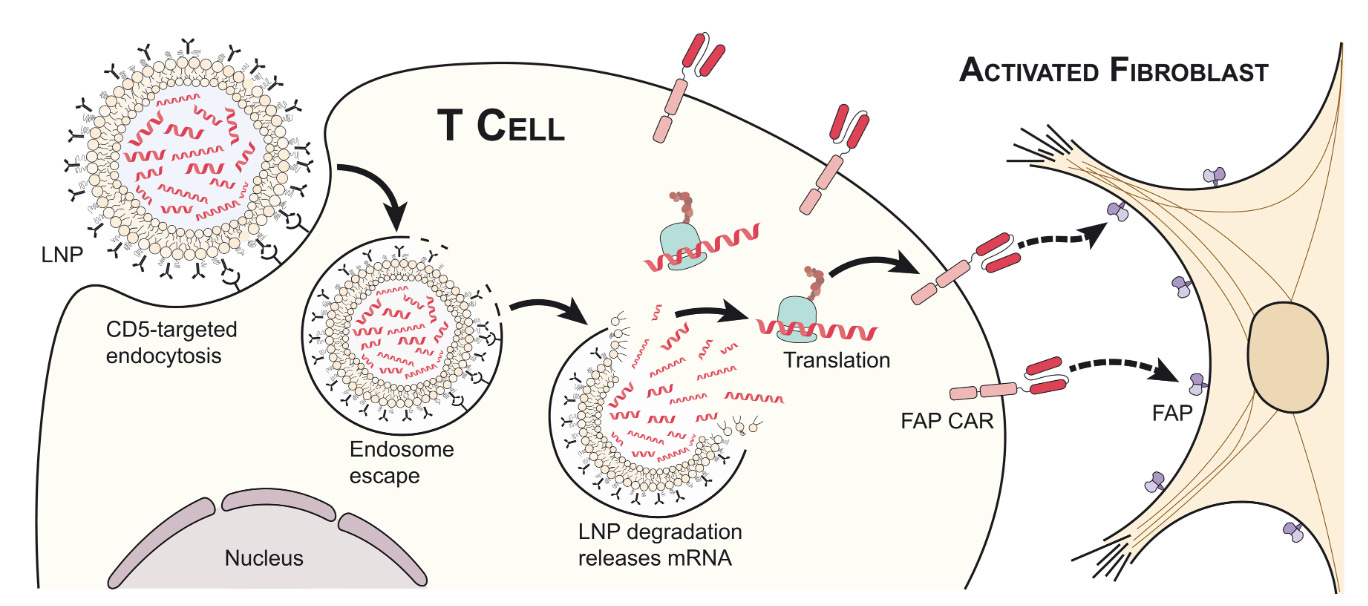
CRISPR and AI aren’t the only technologies that are intersecting with cell therapy. We’ve already successfully scaled mRNA therapies (the recipient of this year’s Nobel Prize) to ship billions of COVID vaccine doses around the world. Given the manufacturing challenges with ex vivo cell therapy and the considerable scientific risk still surrounding the allogeneic alternatives, what if we just used mRNA to program immune cells in vivo? There’s mounting scientific evidence that this might actually work. In a dream team collaboration for the ages, several top researchers from Penn including Carl June and Drew Weissman (the co-recipient of the mRNA Nobel) injected mice with a dose of mRNA containing the instructions to produce a CAR-T receptor. The CAR was designed to bind to the FAP molecule on the surface of fibroblasts in order to destroy scarring of the heart called cardiac fibrosis. The dosed mice presented with reduced scarring. Already, a wave of in vivo cell therapy companies are gearing up to enter the clinic.
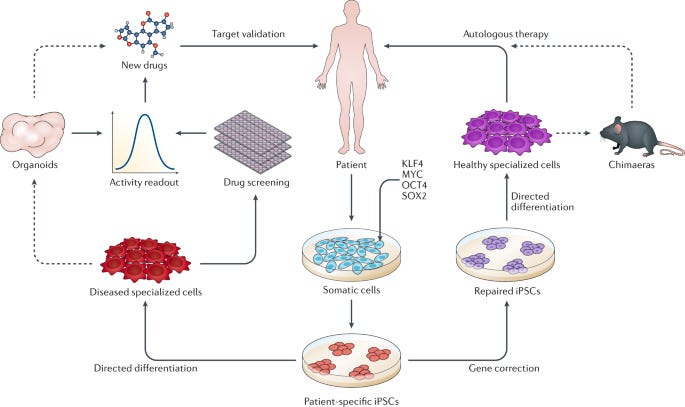
Between efforts in next-generation manufacturing and designs, off-the-shelf cells, and in vivo genetic reprogramming, immune engineering is going through a renaissance. All of these new directions represent a single decade of progress. Another decade from now, the state-of-the-art could look completely different. Still, immune cells are only a single facet of the broader cell-based therapy revolution. In modern biotech, analogies to computer science aren’t metaphorical. The induced pluripotent stem cell (iPSC) revolution makes this crystal clear. iPSC technology takes advantage of the beautiful nature of human development. We all begin life as a single cell and undergo genetically programmed rounds of cell division. Most cells differentiate into a final cell type, like skin, lung, heart, and brain cells. Some cells don’t. These cells—called stem cells—occupy certain niches in the body, and can be stimulated to differentiate into multiple cell types, like a versatile back-up player. In 2006, a research paper from the lab of a Japanese stem cell scientist named Shinya Yamanaka demonstrated a result that bordered on absurd: using a tiny cocktail consisting of only four genes, it’s possible to reprogram differentiated cells into “pluripotent” stem cells that can be differentiated into other cell types. Think about that. Your skin cell, transformed into a neuron. Or muscle cells. Or heart cells. This simple set of genes called “reprogramming factors” (also referred to as Yamanaka factors) laid the basis for the field of iPSC engineering. Now, scientists around the world are working to perfect the right cellular programs to convert iPSCs into every other cell type in the body. This has two immediate ramifications for medicine:
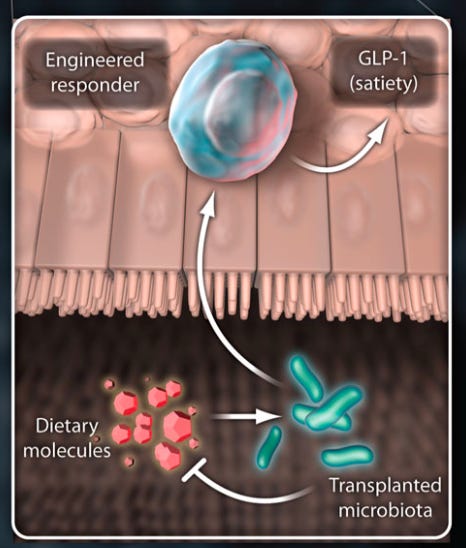
Instead of testing new drugs in mice, why not test them in actual patient-derived cells? Some companies like Rarebase are already partnering with rare disease foundations to rapidly screen libraries of drugs in patient-derived iPSC models. Other companies like bit.bio are working to ship iPSC disease models for every cell type to researchers and companies as a product. They are also partnering with therapeutics companies to produce iPSC-derived cell therapies—including immune cells. Much like for CAR-T therapy, researchers are asking the question: what if we could skip the cell isolation step and reprogram cells in vivo? This approach, called partial reprogramming, has emerged as one of the hottest potential longevity therapies. New longevity companies flush with cash like Retro Biosciences (backed by $180M from Sam Altman), NewLimit (backed by $40M of VC funding and $110M commitments from the founders Brian Armstrong and Blake Byers), and the goliath Altos Labs (launched with $3B in funding—the largest private biotech funding to-date—and a collection of biotech luminaries including Yamanaka) are all racing towards cell reprogramming therapies. Ultimately, this won’t only be for cells, but will be the basis for entire organ systems—the full vision of regenerative medicine. Already, researchers in Israel have produced stem cell derived embryo models all the way up to 14 days of development. While this has immediate implications for the future of human reproduction—and companies like Conception are pursuing this application—it also opens the door to differentiating the embryos into other tissues (and potentially organs) with identical genetics to a patient in need of a transplant. Alright… engineered immune cells to kill cancer, and iPSC/reprogramming therapies to cure, well… disease. What about engineering the microbial cells and communities that inhabit the human body? When you first learn that your own cells are outnumbered by your microbial inhabitants, it can be a bit disconcerting. In the most recent estimates, the human body consists of roughly 30 trillion cells—which is a lot, for sure—but not as many as the 38 trillion bacteria that live inside us. This vast ecosystem of cells is collectively referred to as the human microbiome. Much like the stages of grief, it’s possible to move from a place of fear—or even disgust—about this reality towards a place of awe, and even a reconceptualization of what it means to be a human. We all contain multitudes. More pragmatically, it opens up an entirely new strategy for interfacing with diseased human cells. So far, the field of microbiome therapeutics has had a mixed track record. First met with extreme hype and a lot of funding, many of the first companies have experienced clinical setbacks. Trials have been placed on hold for safety concerns. Companies have died. Some of the early science has been called into question. Questions have been raised about the ability to produce consistent and reliable medicines, given that the first microbiome therapies have been sourced from human poop—a procedure called a fecal microbiota transplant. Seriously. Despite the shitty start, there are signs that microbiome therapies are starting to reach a higher technology readiness level. To start, the FDA approved the first oral microbiome therapy in April earlier this year. Produced by the Cambridge-based Seres Therapeutics, the therapy (called Vowst) contains purified spores from a strain of bacteria capable of outcompeting another strain called C. difficile that can cause a serious gut infection. The fact that Vowst is going to be co-commercialized by Nestlé indicates that it is ready to be scaled. Name another company that ships top-notch espresso capsules, chocolates, and oral microbiome therapies. I’ll wait. Closer to the frontier, things are getting even more sophisticated. It’s long been a goal to move beyond working with individual strains of bacteria. In the “third pillar” paper, the authors wrote: “In the future, the microbial community to be transplanted is likely to be an artificial, well-characterized mixture of strains.” Why? You don’t want uncharacterized poop, but using only a single bacteria is rate-limiting for complexity. A decade later, this is becoming a reality. In a massive effort from several top microbiome labs at Stanford, researchers created a stable synthetic community of transplantable microbes containing over 100 different organisms. Michael Fischbach, one of the “third pillar” authors, was a senior author on this new study. Talk about calling your shots. This type of synthetic microbiome will enable much deeper mechanistic science and more rationally engineered microbiome therapies. A stable synthetic microbiome opens the door to some of the more futuristic ideas like engineered bugs that sense and respond to their environment, and even produce small molecules or biologics directly in the body. Given the mega-blockbuster sales of the first GLP-1 drugs (like semaglutide) for diabetes and obesity, this gets even more interesting. While semaglutide has been forecasted to potentially produce $300B in annual sales, the first generation of medicines has been shown to introduce the risk of severe stomach complications. For some of these medicines, up to 70% of patients experience serious side effects. A logic-gated microbial therapy that reduces stomach complications by tuning GLP-1 production in response to the gut environment could become a blockbuster cell therapy like nothing we’ve ever seen before.
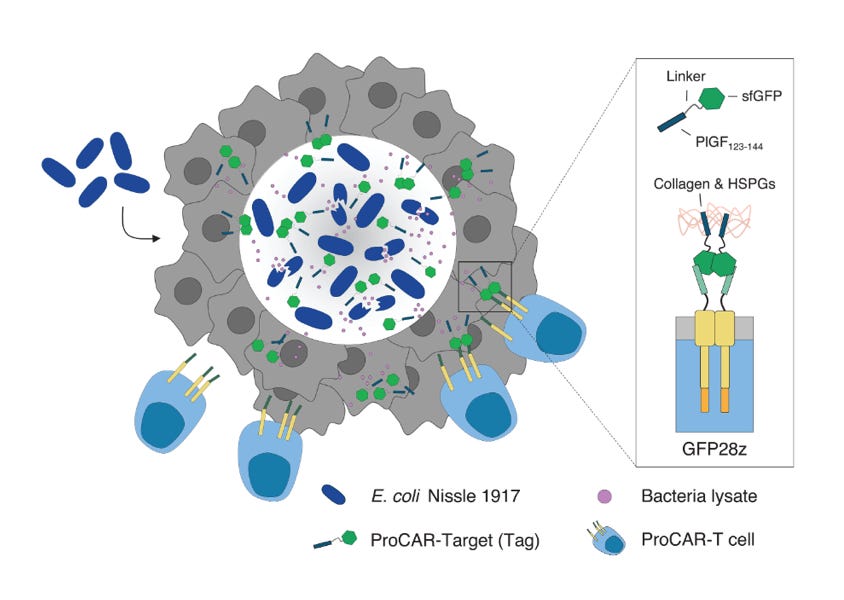
This isn’t the only way that microbial and human cells could be used in combination. As if Dr. William Coley’s research wasn’t already vindicated enough, engineered microbes are now being used to boost CAR-T therapy. The mechanism is absurdly cool. Bioengineers from Columbia University engineered a strain of nonpathogenic E. coli to infiltrate tumors and deliver molecular “tags” that mark the cancer for detection and destruction by CAR-T cells.
Clearly, we’ve come a long way from Coley’s first toxin injections. We’re now using the toolbox of modern biotech to engineer sophisticated cells—and combinations of cells—for a potential curative effect across a wide range of diseases. These drugs are “part drug and part device,” and are like nothing we’ve ever seen before in medicine. So, what’s my point? Even with all of this progress, I think we’re still in the earliest innings for cell-based therapy. Let’s think back to the analogy of medicine’s three pillars:
CAR-T does represent an entirely new type of medicine. But the GLP-1 example illustrates that there could be real benefits to having cells produce big molecules based on smart detection of signals inside the body. Couldn’t this also logically extend to small molecules? After all, some of our best small molecules are things that we discovered being produced by cells in the first place. So, in time, everything could become a subset of cell therapy:
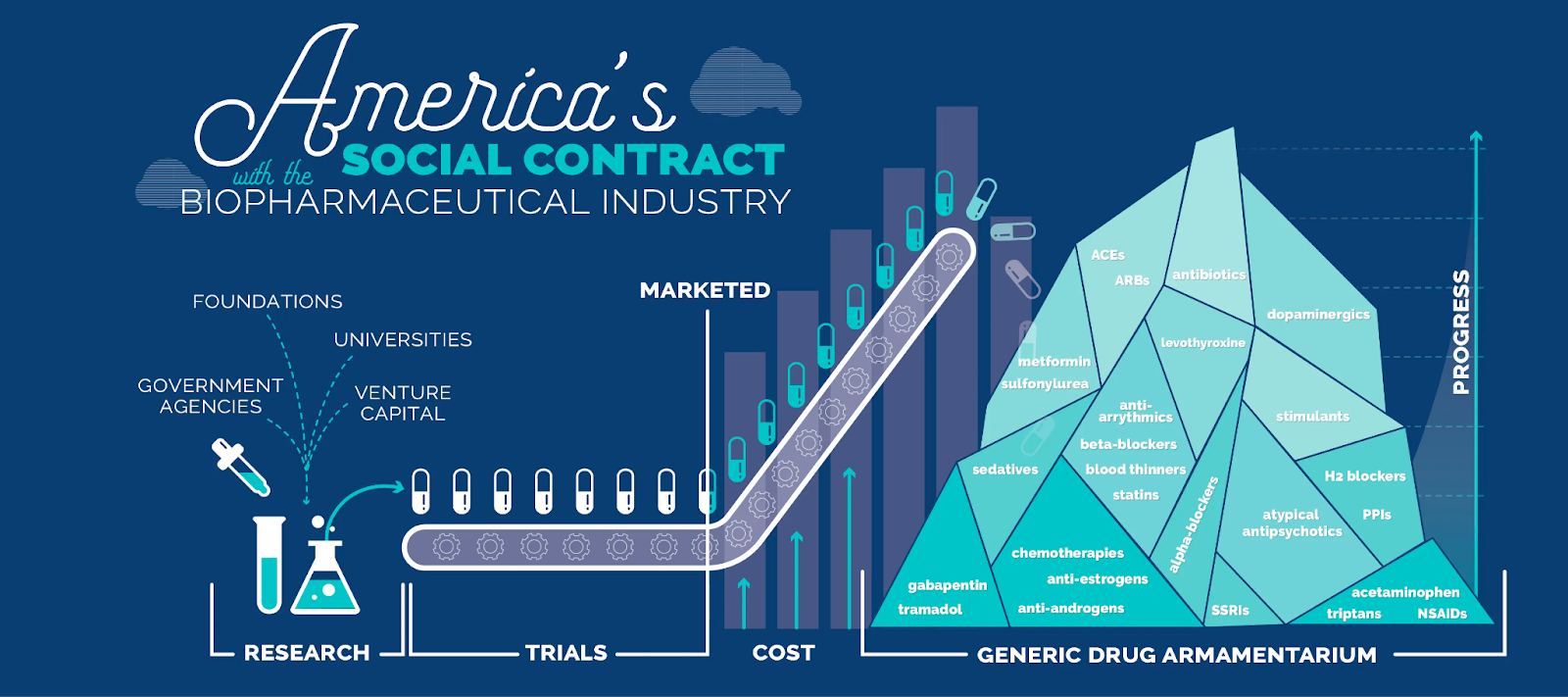
Like we now look back in horror that people used to smoke on planes, our future ancestors could be shocked that this wasn’t always the status quo in medicine. “You’re telling me that for contraception, women used to take oral doses of sex hormones that have widespread effects throughout the body and brain instead of engineering commensal vaginal bacteria to secrete computationally designed proteins that directly obstruct egg cells??? Sure grandma, let’s get you to bed.” The list goes on. “You used to treat your diabetes with an insulin pump and an elaborate dosing schedule instead of islet cell therapy? Okay grandpa…” This is why, in the limit, cells are likely to be Medicine’s Endgame. At this point, we’re solidly in the realm of speculation. Many current medicines already work so well that fancy programmable cell-based delivery wouldn’t be justified. Achieving a future with ubiquitous cellular medicines is an ambitious and long-term project. It’s why my newsletter is called The Century of Biology, not The Decade of Biology. Still, as we gain mastery of cell biology and engineering, large swathes of medicine are up for grabs. We’re on the cusp of a transition towards truly preventative, curative, and even regenerative approaches to treating disease. If biotech continues to head this direction, it opens up important economic questions. For decades, Irv Weissman, one of the pioneers of stem cell biology, has passionately argued that curative one-time cell therapies run counter to the interests of Big Pharma companies, which introduces a serious barrier to transforming medicine. They just want to keep selling you pills! In part, Weissman formed this viewpoint after one of the therapies he developed was canceled during the merger between Sandoz and CIBA to form Novartis—the same company would ultimately license CAR-T therapy from Penn for further development and approval. I think that we now have sufficient evidence that Big Pharma companies are very interested in developing cell therapies. Over time, we may actually run into the opposite problem: cell therapies may produce too much value for Big Pharma. Right now, innovation in drug discovery is maintained by a tenuous balance between society, insurance companies, and drug developers that Peter Kolchinsky of RA Capital refers to as the Biotech Social Contract. We collectively invest in basic research, which is then commercialized, patented and marketed by private companies. But small molecule drugs are information products—all of the value comes from the precious intellectual property (IP), not the actual chemicals, which are commodities worth very little. As a result, when drug patents expire, prices rapidly plummet and society gets access to the medicine cheaply for the rest of time. It’s a pretty good deal. As Kolchinksy would put it, high branded drug prices are “like paying a mortgage, not rent.” After the upfront investment, we all own this generic drug armamentarium forever.
But how do you produce the generic version of a complex cell therapy? Even after the patents expire, the only companies able to produce these medicines will be the ones that invested in the manufacturing and process infrastructure to produce the branded versions of the therapy. How will new entrants compete against the Scale Economies of large cell manufacturing facilities? Who will emerge to compete with Novartis to drive down the price of Kymriah from $475,000 a dose? This is why new approaches to manufacturing and cell therapy delivery are utterly essential. Cell-based cures for cancer, diabetes, and even aging are the potential reward for our continued investment in modern biotechnology. They represent a real opportunity to meaningfully reduce human suffering. To make these treatments universally available, we’ll also need to scale our entire infrastructure layer for biomanufacturing. The scaling of nucleic acid vaccines during the COVID pandemic showed us that this is possible. Now, we need to do the same for cells. We just went on a whirlwind tour of the past, present, and future of cell-based medicine. Hopefully it’s clear that the potential here is enormous. Our growing arsenal of engineered immune cells, stem cells, and microbial cells could redefine our relationship with disease. Each therapeutic invention introduces complex new problems to solve, but if we can realize the full potential of cell-based therapy, medicine will never look the same. Thanks to Dan for editing and to Elliot for sharing his genius. That’s all for today. We’ll be back in your inbox with the Weekly Dose on Friday. Thanks for reading, Packy |
Older messages
Age of Miracles
Friday, October 27, 2023
Launching Season 1: Nuclear Energy
Weekly Dose of Optimism #64
Tuesday, October 24, 2023
Age of Miracles, Solar Resolution, Sturgeon, Purple Cloth, Charles Feeney, Loom
Weekly Dose of Optimism #65
Friday, October 20, 2023
Peace in the Middle East, Techno-Optimism, Scaling Nuclear, Collisons, Direct File
Riskophilia
Thursday, October 19, 2023
Learning to Love Risk
Weekly Dose of Optimism #63
Friday, October 6, 2023
Malaria Vaccine, Nobel Prizes, Focused Ultrasound, Poverty Reduction, Safe Superintelligence, Boats
You Might Also Like
🔮 $320B investments by Meta, Amazon, & Google!
Friday, February 14, 2025
🧠 AI is exploding already!
✍🏼 Why founders are using Playbookz
Friday, February 14, 2025
Busy founders are using Playbookz build ultra profitable personal brands
Is AI going to help or hurt your SEO?
Friday, February 14, 2025
Everyone is talking about how AI is changing SEO, but what you should be asking is how you can change your SEO game with AI. Join me and my team on Tuesday, February 18, for a live webinar where we
Our marketing playbook revealed
Friday, February 14, 2025
Today's Guide to the Marketing Jungle from Social Media Examiner... Presented by social-media-marketing-world-logo It's National Cribbage Day, Reader... Don't get skunked! In today's
Connect one-on-one with programmatic marketing leaders
Friday, February 14, 2025
Enhanced networking at Digiday events
Outsmart Your SaaS Competitors with These SEO Strategies 🚀
Friday, February 14, 2025
SEO Tip #76
Temu and Shein's Dominance Is Over [Roundup]
Friday, February 14, 2025
Hey Reader, Is the removal of the de minimis threshold a win for e-commerce sellers? With Chinese marketplaces like Shein and Temu taking advantage of this threshold, does the removal mean consumers
"Agencies are dying."
Friday, February 14, 2025
What this means for your agency and how to navigate the shift ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏
Is GEO replacing SEO?
Friday, February 14, 2025
Generative Engine Optimization (GEO) is here, and Search Engine Optimization (SEO) is under threat. But what is GEO? What does it involve? And what is in store for businesses that rely on SEO to drive
🌁#87: Why DeepResearch Should Be Your New Hire
Friday, February 14, 2025
– this new agent from OpenAI is mind blowing and – I can't believe I say that – worth $200/month